You Are Leaving Avistone's Website
You have selected a link that will take you to a site maintained by a third party. Avistone provides this link as a service to website visitors. Avistone is not responsible for the privacy policy of any third party websites. We encourage you to read the privacy policy of every website you visit. Click 'Cancel' to return to Avistone's site or 'Continue' to proceed.
Avistone Presents Latest Clinical Studies of Andamertinib (PLB-1004) in Advanced NSCLC with EGFR Exon 20 Insertions at 2025 ESMO
The 2025 European Society for Medical Oncology (ESMO) Congress is held in Berlin, Germany, from October 17 to 21 (local time). Beijing Avistone Biotechnology Co., Ltd. ("Avistone") announced the latest study results of Andamertinib (PLB-1004) in advanced non-small-cell lung cancer with EGFR exon 20 insertions after platinum-based chemotherapy or immunotherapy at this year's congress.
01 Congress Poster
Poster Title:
PLB-1004 in Advanced Non-Small-Cell Lung Cancer with EGFR Exon 20 Insertions After Platinum-Based Chemotherapy or Immunotherapy: Results from the Multicenter, Multicohort, Single-Arm Phase 2 KANNON Study
Abstract Number: 5120
Principal Investigators: Yilong Wu (Guangdong Provincial People's Hospital), Jinji Yang (Guangdong Provincial People's Hospital)
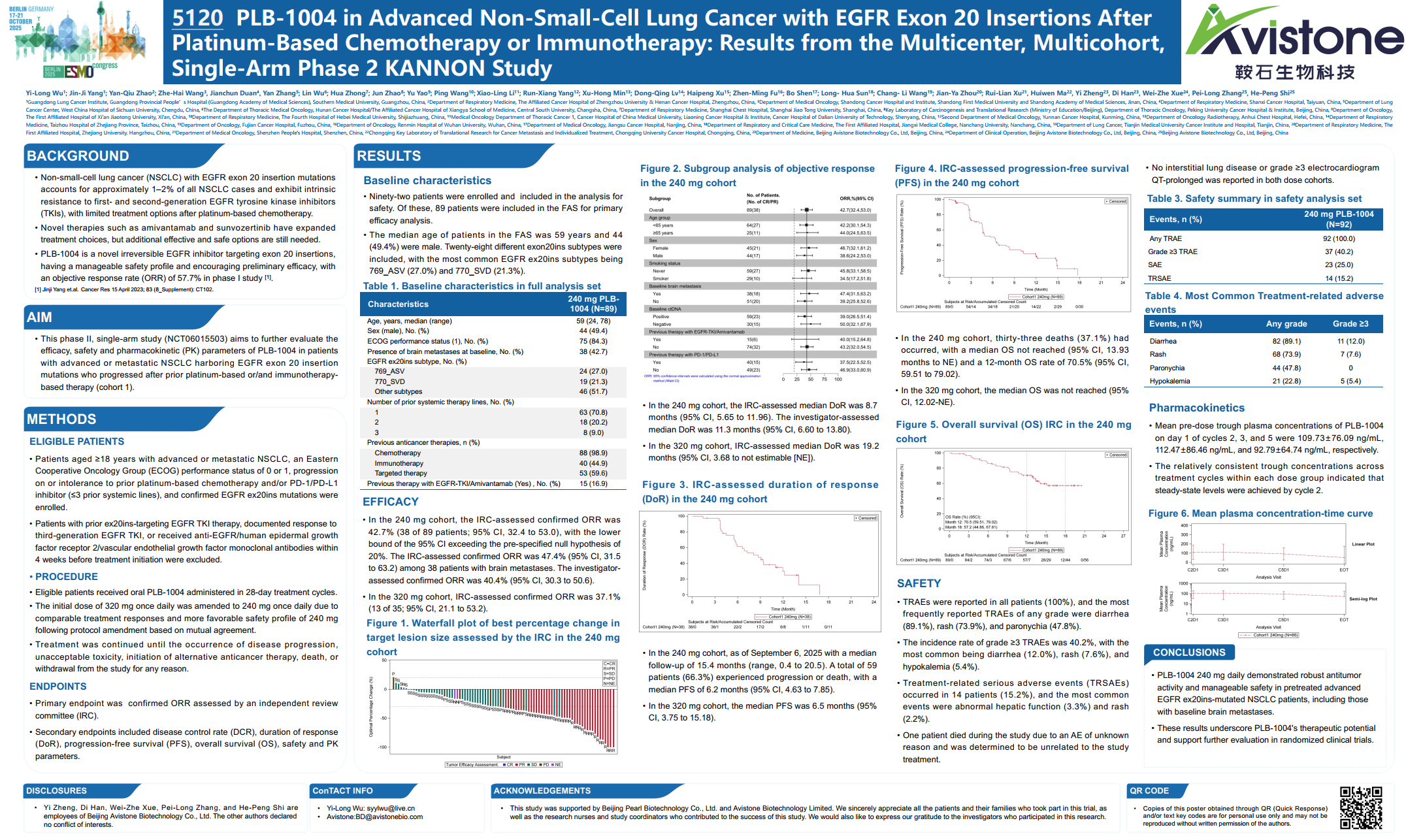
NSCLC with EGFR exon 20 insertion mutations accounts for approximately 1-2% of all NSCLC cases and exhibits intrinsic resistance to first- and second-generation EGFR tyrosine kinase inhibitors (TKIs), with limited treatment options after platinum-based chemotherapy[1]. Results from the KANNON study confirm that Andamertinib (PLB-1004) demonstrates significant clinical benefit and tolerable safety in advanced NSCLC patients with EGFR exon 20 insertions after platinum-based chemotherapy or immunotherapy.
A total of 92 patients were enrolled in the KANNON study, with 89 patients included in the Full Analysis Set (FAS) for primary efficacy analysis. The treatment regimen was 240mg orally once daily (QD). As of September 6, 2025, the Independent Review Committee (IRC)-confirmed overall response rate (ORR) was 42.7%, the median duration of response (mDOR) was 8.7 months (11.3 months as assessed by investigators). The IRC-assessed median progression-free survival (mPFS) was 6.2 months, the median overall survival (mOS) was not reached, and the 12-month OS rate was 70.5%. Subgroup analysis showed that among 38 patients (42.7% of the total) with baseline brain metastases, the IRC-confirmed ORR was 47.4%. In terms of safety data, the incidence of grade ≥3 treatment-related adverse events (TRAEs) was 40.2%. The most common grade ≥3 TRAEs were diarrhea (12.0%), rash (7.6%), and hypokalemia (5.4%). No interstitial lung disease or grade ≥3 QT interval prolongation on electrocardiogram was reported.
02 About Andamertinib (PLB-1004) Capsules
Andamertinib (PLB-1004) Capsules is a China-developed epidermal growth factor receptor (EGFR) small-molecule inhibitor with global intellectual property rights. It features high selectivity and can cross the blood-brain barrier. Preclinical studies have shown that it can effectively and irreversibly target EGFR exon 20 insertion mutations (EGFR ex20ins). In addition, this molecule can also effectively target other EGFR mutation types such as Del19, L858R, and T790M, with high selectivity.
The New Drug Application (NDA) for Andamertinib was accepted by China's National Medical Products Administration (NMPA) in May 2025 and was included in the priority review and approval process. The proposed indication: for the treatment of patients with locally advanced or metastatic NSCLC who have disease progression during or after prior platinum-based chemotherapy and/or PD-1/PD-L1 immunotherapy, or are intolerant to such treatments, and have confirmed EGFR exon 20 insertion mutations through testing.
References:
1.Chouaid C, Filleron T, Debieuvre D, et al. A Real-World Study of Patients with Advanced Non-squamous Non-small Cell Lung Cancer with EGFR Exon 20 Insertion: Clinical Characteristics and Outcomes. Target Oncol. 2021;16:801-811.
Forward-Looking Statements
The forward-looking statements made in this article relate only to events or information as of the date on which such statements are made in this article. Except as required by law, after the date of making the forward-looking statements, we shall have no obligation to update or publicly revise any forward-looking statements or unforeseen events, regardless of whether new information, future events or other circumstances arise. Please read this article carefully and understand that our actual future results or performance may differ materially from expectations. All statements contained in this article are made as of the publication date of this article and are subject to change in light of future developments.









