You Are Leaving Avistone's Website
You have selected a link that will take you to a site maintained by a third party. Avistone provides this link as a service to website visitors. Avistone is not responsible for the privacy policy of any third party websites. We encourage you to read the privacy policy of every website you visit. Click 'Cancel' to return to Avistone's site or 'Continue' to proceed.
2025 WCLC: Vebreltinib of Avistone: Four Clinical Studies on MET Alterations were Presented as Poster Displays
Author:Jinji Yang et al. Time:2025-09-08
The 2025 World Conference on Lung Cancer (WCLC), hosted by the International Association for the Study of Lung Cancer (IASLC), will be held in Barcelona, Spain, from September 6 to 9 (local time). Avistone will make a major release of its four clinical studies on MET abnormalities in the form of posters.
[Poster 1498]
Efficacy of Vebreltinib in advanced NSCLC patients with MET exon 14-skipping: post-hoc analysis of KUNPENG study
Principal Investigator:Jinji Yang(Guangdong Lung Cancer Institute)
Conference session:P3.12 - Metastatic Non-small Cell Lung Cancer – Targeted Therapy
Time:9/9/2025 10:00:00 AM - 9/9/2025 11:30:00 AM
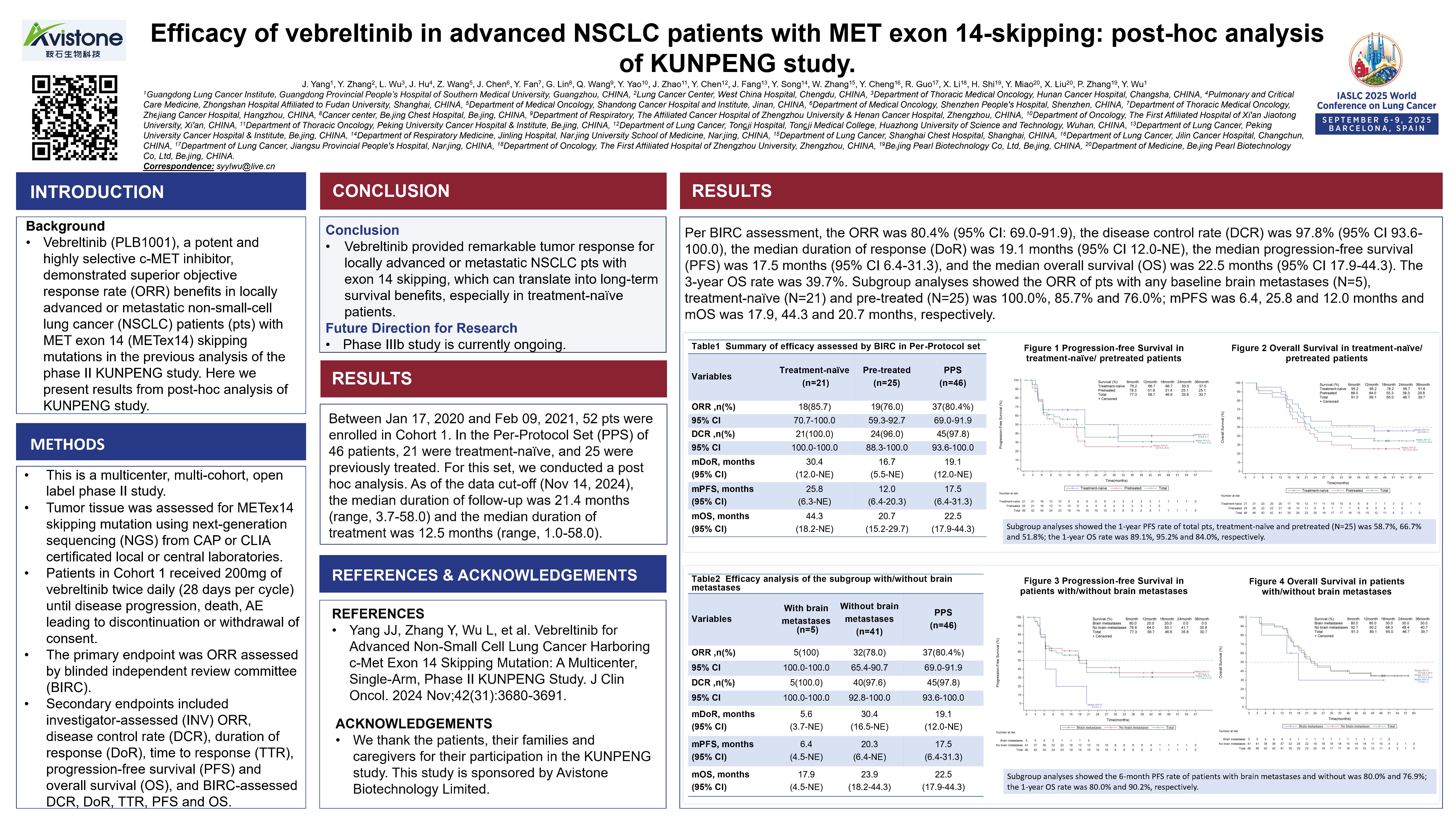
Vebreltinib (PLB1001), a potent and highly selective c-MET inhibitor, demonstrated superior objective response rate (ORR) benefits in locally advanced or metastatic non-small-cell lung cancer (NSCLC) patients (pts) with MET exon 14 (METex14) skipping mutations in the previous analysis of the phase II KUNPENG study. As of November 14, 2024, the Per-Protocol Set (PPS) evaluated by BIRC showed an Objective Response Rate (ORR) of 80.4% (95% Confidence Interval [CI]: 69.0-91.9), a Disease Control Rate (DCR) of 97.8% (95% CI: 93.6-100.0), a median Duration of Response (mDoR) of 19.1 months (95% CI: 12.0-Not Estimable [NE]), a median Progression-Free Survival (mPFS) of 17.5 months (95% CI: 6.4-31.3), a median Overall Survival (mOS) of 22.5 months (95% CI: 17.9-44.3), and a 3-year Overall Survival rate of 39.7%.
[Poster 1521]
A Phase Ib/II study of Vebreltinib plus Tislelizumab in patients with MET-amplified and PD-L1-positive locally advanced or metastatic NSCLC
Principal Investigator:Zhijie Wang(Cancer Hospital, CAMS&PUMC)
Conference session:P3.12 – Metastatic NSCLC – Targeted Therapy
Time:9/9/2025 10:00:00 AM - 9/9/2025 11:30:00 AM
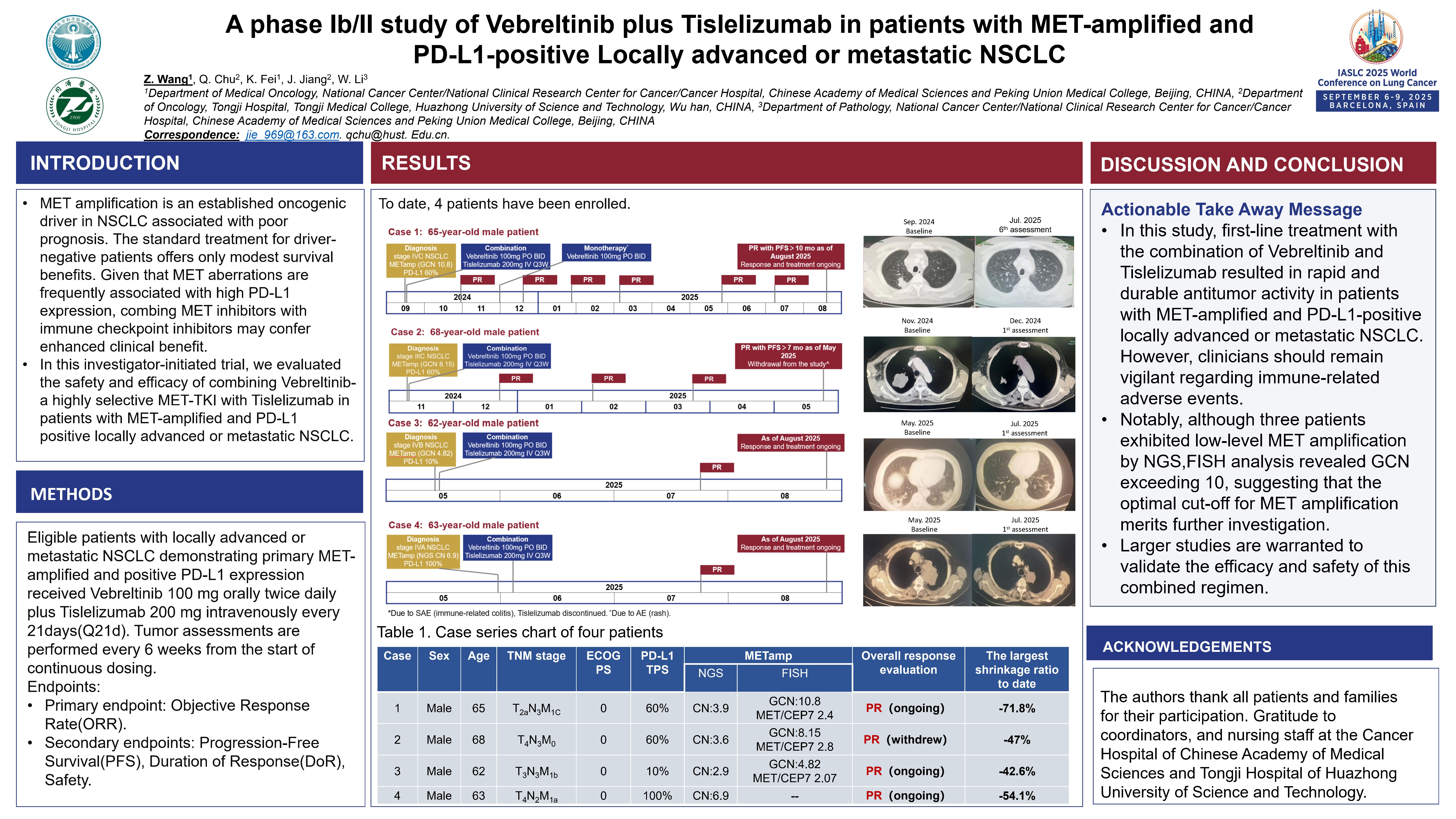 MET amplification is an established oncogenic driver in NSCLC associated with poor prognosis. The standard treatment for driver-negative patients offers only modest survival benefits. Given that MET aberrations are frequently associated with high PD-L1 expression, combing MET inhibitors with immune checkpoint inhibitors may confer enhanced clinical benefit. In this investigator-initiated trial, we evaluated the safety and efficacy of combining Vebreltinib-a highly selective MET-TKI with Tislelizumab in patients with MET-amplified and PD-L1 positive locally advanced or metastatic NSCLC. In this study, first-line treatment with the combination of Vebreltinib and Tislelizumab resulted in rapid and durable antitumor activity in patients with MET-amplified and PD-L1-positive locally advanced or metastatic NSCLC. As of the data publication, 4 patients achieved a partial response (PR), with the maximum tumor shrinkage reaching -71.8%. However, clinicians should remain vigilant regarding immune-related adverse events.
MET amplification is an established oncogenic driver in NSCLC associated with poor prognosis. The standard treatment for driver-negative patients offers only modest survival benefits. Given that MET aberrations are frequently associated with high PD-L1 expression, combing MET inhibitors with immune checkpoint inhibitors may confer enhanced clinical benefit. In this investigator-initiated trial, we evaluated the safety and efficacy of combining Vebreltinib-a highly selective MET-TKI with Tislelizumab in patients with MET-amplified and PD-L1 positive locally advanced or metastatic NSCLC. In this study, first-line treatment with the combination of Vebreltinib and Tislelizumab resulted in rapid and durable antitumor activity in patients with MET-amplified and PD-L1-positive locally advanced or metastatic NSCLC. As of the data publication, 4 patients achieved a partial response (PR), with the maximum tumor shrinkage reaching -71.8%. However, clinicians should remain vigilant regarding immune-related adverse events.
[Poster 2038]
Vebreltinib plus EGFR-TKI in EGFR-mutated NSCLC with MET amplification and/or overexpression after failure on EGFR-TKI:A retrospective study
Principal Investigator:Zhangzhou Huang(Fujian Cancer Hospital)
Conference session:P3.12 - Metastatic Non-small Cell Lung Cancer – Targeted Therapy
Time:9/9/2025 10:00:00 AM - 9/9/2025 11:30:00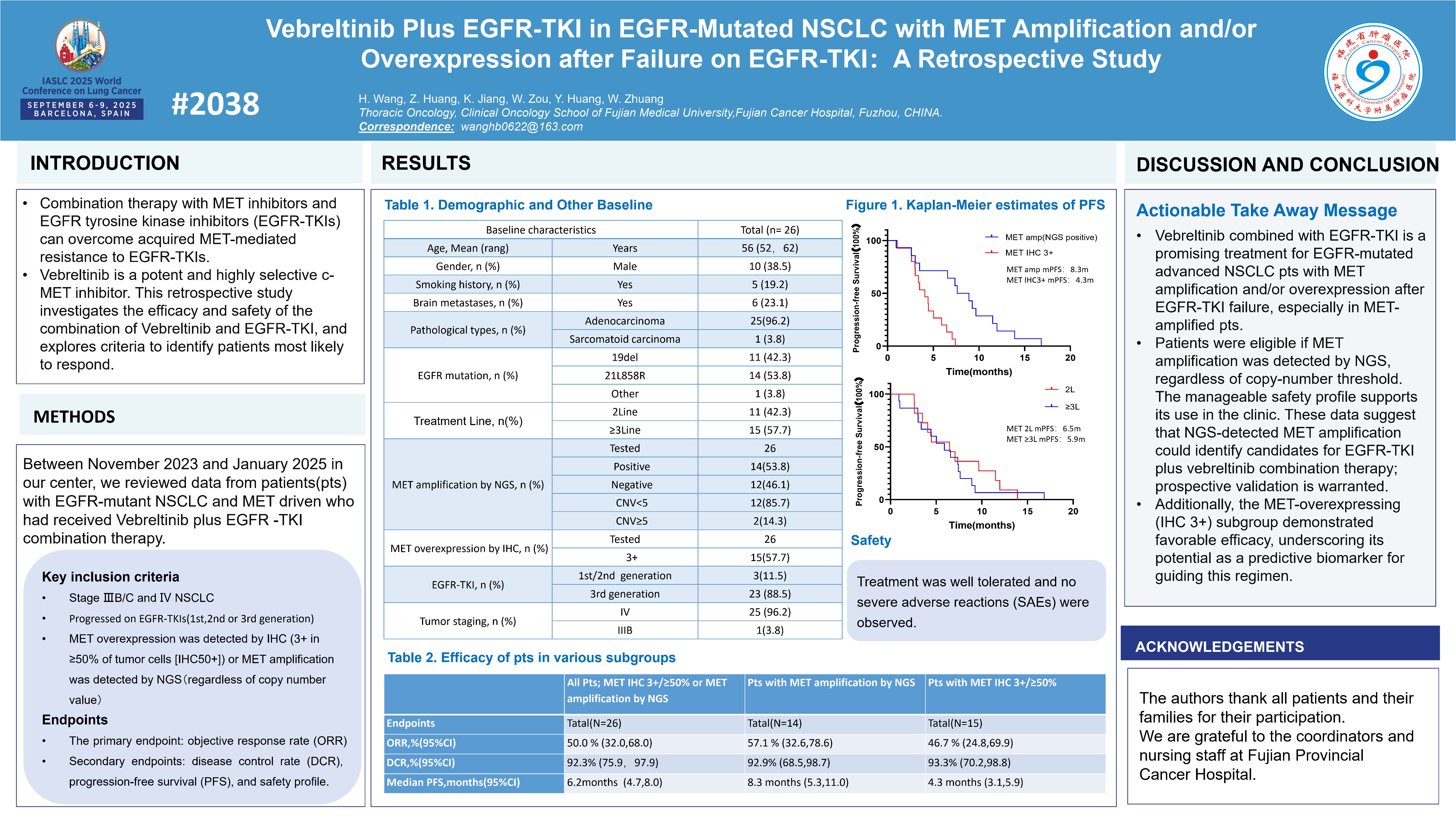
Combination therapy with MET inhibitors and EGFR tyrosine kinase inhibitors (EGFR-TKIs) can overcome acquired MET-mediated resistance to EGFR-TKIs. Vebreltinib is a potent and highly selective c-MET inhibitor. This retrospective study investigates the efficacy and safety of the combination of Vebreltinib and EGFR-TKI, and explores criteria to identify patients most likely to respond. Key inclusion criteria for patients: MET amplification was detected by NGS, regardless of copy number value. Data indicate that MET amplification confirmed by NGS can serve as a basis for selecting candidate patients for the EGFR-TKI combined with Vebreltinib treatment regimen: the objective response rate (ORR) was 50.0% in the total population and the subgroup with MET IHC 3+/≥50% or NGS-verified MET amplification, while the ORR in the subgroup with only NGS-verified MET amplification reached 57.1%. This conclusion still awaits validation by prospective studies.
Furthermore, patients in the subgroup with MET overexpression (defined by an immunohistochemical staining intensity score of IHC 3+ or ≥50% positive cells) demonstrated favorable therapeutic efficacy. The objective response rate (ORR) in the MET IHC 3+/≥50% subgroup reached 46.7%, which highlights the value of MET overexpression as a potential predictive biomarker for guiding this treatment regimen.
[E poster 2773]
Vebreltinib as neoadjuvant therapy in three cases of MET exon 14 skipping mutation-positive NSCLC: A case series
Principal Investigator:Yifei Zhou(Shanghai Pulmonary Hospital)
Conference session:EP.08.Local-Regional Non-small Cell Lung Cancer
Time:Tuesday,September 9,2025 at 3:00 PM CEST/UTC +2
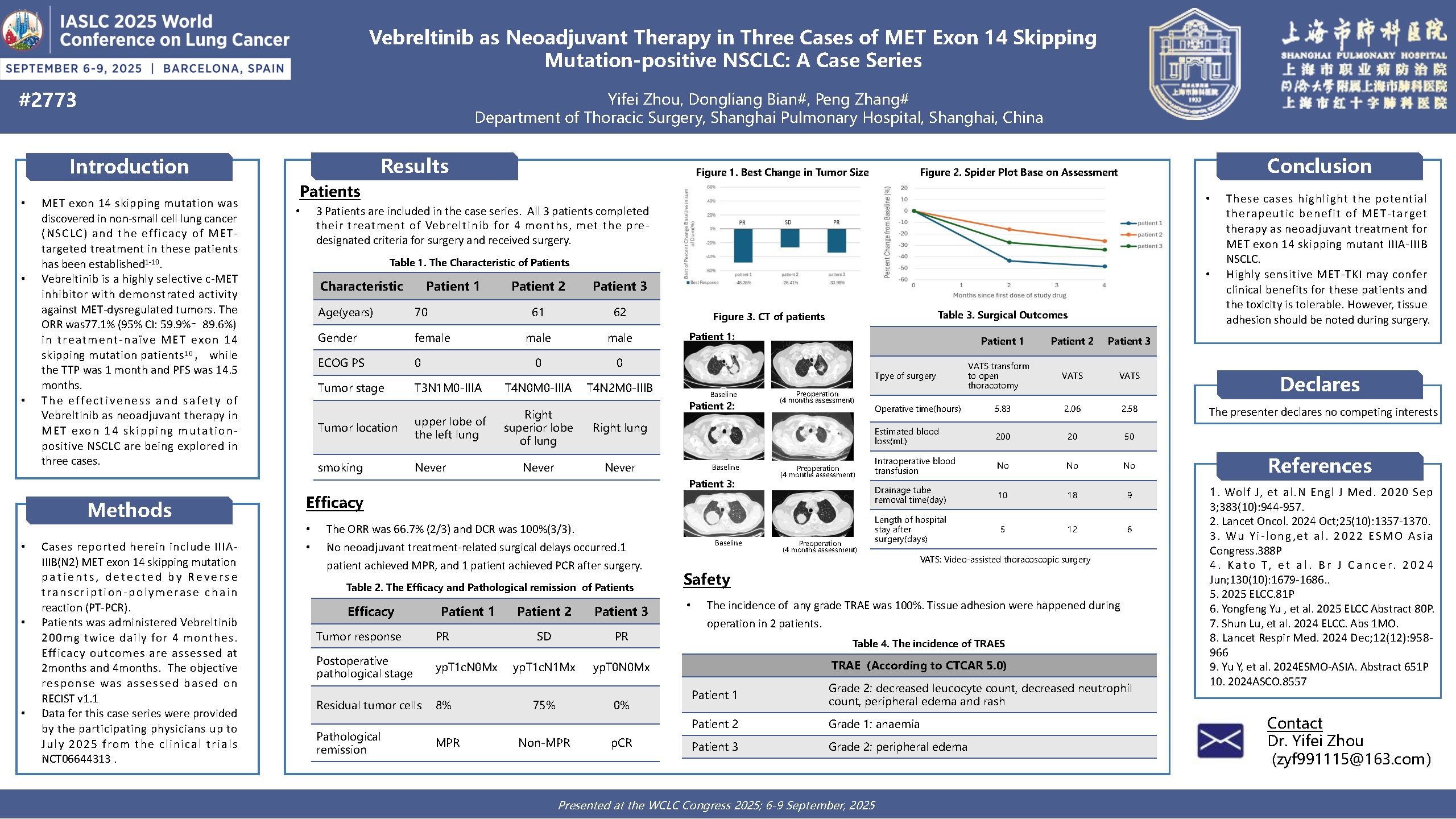
MET exon 14 skipping mutation was discovered in non-small cell lung cancer (NSCLC) and the efficacy of MET targeted treatment in these patients has been established. Vebreltinib is a highly selective c-MET inhibitor with demonstrated activity against MET-dysregulated tumors. The ORR was 77.1%(95% CI: 59.9%∼ 89.6%) in treatment-naïve MET exon 14 skipping mutation patients,while the mTTR was 1 month and PFS was 14.5 months. The effectiveness and safety of Vebreltinib as neoadjuvant therapy in MET exon 14 skipping mutation positive NSCLC are being explored in three cases. These cases highlight the potential therapeutic benefit of MET-target therapy as neoadjuvant treatment for MET exon 14 skipping mutant IIIA-IIIB NSCLC. Highly sensitive MET-TKI may confer clinical benefits for these patients and the toxicity is tolerable. However, tissue adhesion should be noted during surgery[1-10].
References:
1. Wolf J, et al.N Engl J Med. 2020 Sep 3;383(10):944-957.
2. Lancet Oncol. 2024 Oct;25(10):1357-1370.
3. Wu Yi-long,et al. 2022 ESMO Asia Congress.388P
4. Kato T, et al. Br J Cancer. 2024 Jun;130(10):1679-1686..
5. 2025 ELCC.81P
6. Yongfeng Yu , et al. 2025 ELCC Abstract 80P.
7. Shun Lu, et al. 2024 ELCC. Abs 1MO.
8. Lancet Respir Med. 2024 Dec;12(12):958-966
9. Yu Y, et al. 2024ESMO-ASIA. Abstract 651P
10. 2024ASCO.8557
Forward-Looking Statements
The forward-looking statements made in this article relate only to events or information as of the date on which such statements are made in this article. Except as required by law, after the date of making the forward-looking statements, we shall have no obligation to update or publicly revise any forward-looking statements or unforeseen events, regardless of whether new information, future events or other circumstances arise. Please read this article carefully and understand that our actual future results or performance may differ materially from expectations. All statements contained in this article are made as of the publication date of this article and are subject to change in light of future developments.









